Part 2 of a 5 Part Blog Series on | "Advancing Public Health with Wearables; Strategic Development of IoMT Biosensing Lifestyle Devices"
Wearable IoT devices are a massive step forward in the effort to provide high quality, highly personalized care to every patient, offering the freedom to manage chronic illnesses and monitor conditions, without being chained to a healthcare facility. One of two categories of IoMT wearables, biosensory devices collect physical samples from the human body to be analyzed in real-time. While alleviating some of the stress of managing health for patients, they also allow providers to administer preventative care in a much shorter period than previously possible.
Overview of Biosensory Devices
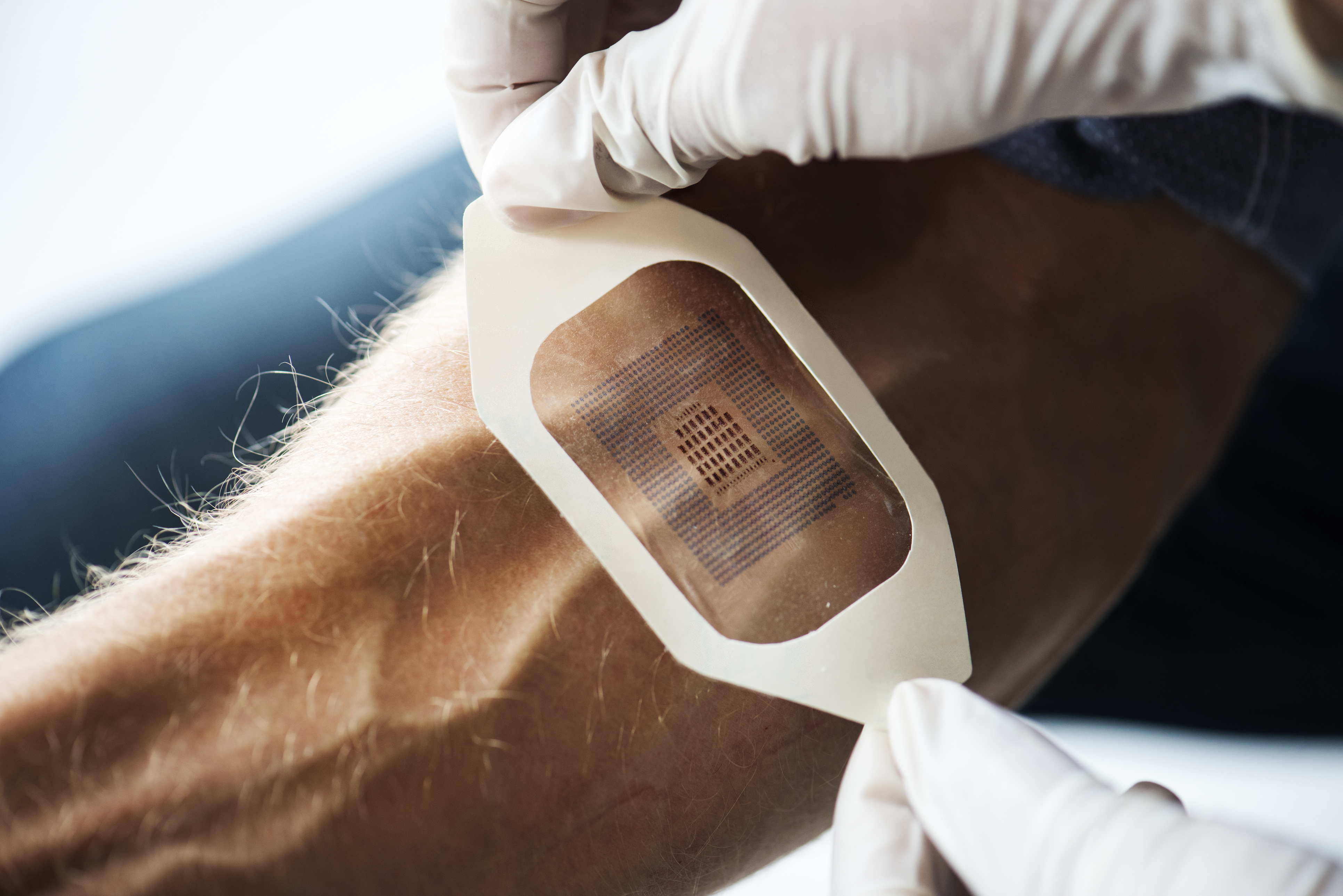
A biosensory device is comprised of two major parts: a transducer and a biological element, or bio-element. For the device to operate, the bio-element must first interact with an analyte it has collected. This results in a biological reaction, which is then converted into an electrical signal via the transducer.
To break this definition down further, we may look at each of these components individually, beginning with the analyte. Analytes are biological samples acquired through either non-invasive or minimally invasive methods, typically consisting of sweat, saliva, tears, breath, or interstitial fluid, which is fluid contained within the body between the blood vessels and cells. Next, the biological element may be classified as part of one of three groups: (1) the biocatalytic group, composed of enzymes, (2) the bioaffinity group, which includes antibodies and nucleic acids, or (3) the microbe-based group, which contains microorganisms. When the analyte and biological element interact, they create a biological reaction that generates a signal that may be electrical, optical, or thermal. The device’s transducer can convert that signal into a measurable electrical parameter, i.e., current and voltage. When combined, these interactions form the basis of a biosensory device whose clinical objective is to compare collected data to known biomarkers, indicating a condition or chronic disease and the body’s response to continued treatment of such.
Challenges of Development and Commercialization
With telemedicine and telediagnostic technologies currently in high demand, biosensory wearable form factors are becoming increasingly popular. Yet, like any innovative technology, there are challenges to developing and commercializing such devices. An example of one such challenge is establishing a correlation between biomarkers found in blood samples (AKA Golden Standard) and those found in externally accessible biosample analytes, which requires a considerable amount of validation work. Biomarkers derived from tears and interstitial fluid typically correlate well with blood biomarkers, while sweat biomarkers need further studies to prove correlation. While saliva does correlate well with blood biomarkers, samples contain low concentrations of biomarkers and external factors such as food particles or other biofouling materials may influence testing. Another common challenge with wearable biosensory devices is encouraging proper use and compliance by prioritizing users’ comfort while still making direct contact with the body and biosample. Lifestyle, human factors engineering optimization can promote this compliance, while advanced materials and intelligent ergonometric design may provide the necessary scalability (i.e., to human form) and flexibility for lifestyle motion. Challenges may also arise when transporting small quantities of biosamples to the reaction zone via microfluidic manifolding; in these cases, sample conservation and evaporation mitigation techniques must be implemented.
Examples of Applications
As a rapidly growing area of interest, there are several examples of biosensory devices to be found currently in development throughout the industry. Integrated wearable sensor array bands are now being applied to the wrist and utilized in multiplexed sweat extraction and analysis, paired with a sensor array configuration. With this device, sweat is induced via electrical stimulation (eliminating the need for strenuous exercise), which is then analyzed to provide simultaneous detection of chloride, sodium, and glucose. In another device configuration chemical-electrophysiological hybrid biosensor configurations provide real-time health and fitness monitoring utilizing textiles featuring screen-printed electrodes worn on the body. An oral device format accommodates a mouthguard-based wearable salivary uric acid biosensing platform integrated with wireless electronics to analyze uric acid concentrations.
Yet, wearable IoMT biosensory applications have a broad reach and may benefit patients in various cases. For example, continuous Glucose Monitoring (CGM) and cholesterol monitoring are commonly performed with IoMT wearables.
Biosensory devices offer providers the opportunity to receive and monitor lab-quality data at any time, making possible higher quality and more personalized healthcare. This ability can be instrumental in preventing needless worsening of conditions when patients require around-the-clock care, reducing waiting periods between when symptoms appear and when a patient may see their provider. Importantly, biosensor technology may also be combined with physiological technology to produce a robust dataset that offers a more comprehensive picture of the patient’s condition.
Keep an eye out for our next blog, where we will dive deep into wearable IoMT Physiological Devices and demonstrate how these devices are commercialized under MIDI’s Innovation Roadmap™.
here…